orbital notation for chlorine|chlorine electron configuration excited state : Cebu That is, the orbital notation of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed . Latest events select. Soccer. Scrollbar
PH0 · orbital notation for cl
PH1 · orbital energy diagram for chlorine
PH2 · ground state electron configuration for chlorine
PH3 · give the full electron configuration for sulfur
PH4 · electron configuration for calcium
PH5 · complete the electron configuration for p
PH6 · complete electron configuration for chlorine
PH7 · chlorine electron configuration excited state
PH8 · Iba pa
How is the business class experience on Asiana Airlines A350? Find out in this detailed review of the flight from Seoul to Frankfurt, featuring the seat, food, entertainment, and service. .upresults.nic.in 2024 Result Link: यूपी बोर्ड 10वीं, 12वीं का रिजल्ट घोषित, यहां रोल नंबर डालकर चेक .
orbital notation for chlorine*******To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number of electrons for the Cl atom (there.In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the .In the chlorine ground-state electron configuration, the five electrons of the 3p orbital are located in the p x (2), p y (2), and p z (1) orbitals. Then the correct electron .That is, the orbital notation of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed .
For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 electrons in chlorine atom). When .Chlorine orbital diagram. The orbital diagram of chlorine shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s .
BlockElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .2p has 3 orbitals, and therefore can hold max of 3 x 2 = 6 electrons; For energy level 3, there are 3 sublevels, 3s, 3p and 3d. 3s, has 1 orbital, therefore, max 2 electrons. 3p, .The p orbitals are px, py, and pz, and if represented on the 2p energy with full orbitals would look like: 2p x 2 2p y 2 2p z 2. The expanded notation for neon (Ne, Z=10) is .
orbital notation for chlorineSuch overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more .They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1s22s22p6 part of the configuration.
The Chlorine orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining five electrons in the 3p orbital. The orbital diagram for a ground-state electron configuration of a Chlorine atom is shown below-. Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons .
For example, chlorine is in group 7, so it has seven valence electrons. Indeed, we can write its electron configuration as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5 \rm 1s^22s^22p^63s^23p^5 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5. As we can see, chlorine has seven electrons in the 3 s \rm 3s 3s and 3 p \rm 3p 3p orbitals, which are the valence electrons. According to Hund’s rule, this arrangement of electrons into their orbitals states that the lowest energy atomic orbital should fill first. Let us see the electronic configuration of chlorine. Electronic Configuration Chlorine electron configuration notation. Cl electron configuration notation is 1S 2 2S 2 2P 6 3S 2 3P 5. This notation .
The electron configuration for phosphorus is 1s 2 2s 2 2p6 3 s2 3p3 and the orbital diagram is drawn below. 1.4: Electron Configurations and Electronic Orbital Diagrams (Review) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom indicates the number of valence . Electron configuration of Chlorine (Cl) [Ne] 3s 2 3p 5: 1s 2 2s 2 2p 6 3s 2 3p 5: 2, 8, 7: 18: Electron configuration of Argon (Ar) [Ne] 3s 2 3p 6: 1s 2 2s 2 2p 6 3s 2 3p 6: 2, 8, 8: 19: Electron configuration of Potassium (K) [Ar] 4s 1: . Orbital Diagram of All Elements (Diagrams given Inside) Subscribe to our newsletter. Write the noble gas configuration by writing the noble gas core, followed by the valence electrons. A noble gas core is the noble gas element symbol enclosed in brackets: [He], [Ne], [Ar], [Kr], [Xe], or [Rn]. The valence electrons are “leftover” electrons that don’t fill a shell or satisfy the octet rule (except for noble gases) or 18 .Uses and properties. Image explanation. The symbol shows a gas mask. This is because chlorine is a toxic gas, and has been used as a chemical weapon. Chlorine is yellowy-green in colour, as is the image. Appearance. A yellowy-green dense gas with a choking smell. Uses. Chlorine kills bacteria – it is a disinfectant.We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. The neutral atom chlorine (Z=17), for instance has 17 electrons. Therefore, its ground state electronic configuration can be written as 1s 2 .orbital notation for chlorine chlorine electron configuration excited state Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. . Chlorine (Cl) 18: Argon (Ar) 19: Potassium (K) 20: Calcium (Ca) 21: Scandium (Sc) 22: Titanium (Ti) 23: Vanadium (V) 24: Chromium (Cr) .
Hint: In order to write condensed orbital notation of electronic configuration of the given elements, we must know how the electrons are filled in the atomic orbitals and how the subshells are arranged. The electrons are arranged in the subshell according to Aufbau's principle. Complete Solution : Each electron present in an atom is present in a .
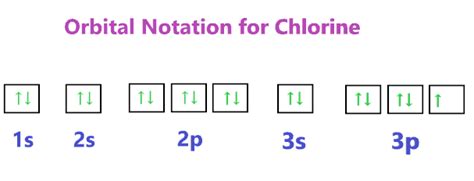
The electronic configuration of a ground state chlorine is [Ne]3s 2 3p 5 (1s 2 2s 2 2p 6 3s 2 3p 5 ). This means that the 4s, 3d and 4p orbitals shown are unoccupied; thus their extent and energy are somewhat different than would be found for orbitals with electrons in them. The orbitals appear in the pull-down menus from lowest (at top) to .
chlorine electron configuration excited state The electronic configuration of a ground state chlorine is [Ne]3s 2 3p 5 (1s 2 2s 2 2p 6 3s 2 3p 5 ). This means that the 4s, 3d and 4p orbitals shown are unoccupied; thus their extent and energy are somewhat different than would be found for orbitals with electrons in them. The orbitals appear in the pull-down menus from lowest (at top) to .For this example, we will be using Chlorine. To allow space for the next step, draw the orbitals further apart than you would for an atomic orbital diagram. In the second (center) column, draw lines above and below each orbital. The bottom line(s) represent the bonding orbital(s), and the top line(s) represent the antibonding orbital(s).
The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell ( 2s) has 1 electron. Figure 2.7.1 2.7. 1: Shell diagrams of hydrogen (H), helium (He), lithium (Li), and Berryellium (Be) atoms. (CC BY-SA 2.0 UK; Greg Robson modified by Pumbaa via Wikipedia)
The ground state configuration of the neutral chlorine atom is 1s^2 2s^2 2p^6 3s^2 3p^5 The ground state is the most stable energy state for the electrons of a given atom A neutral chlorine atom has 17 protons (atomic number 17) and 17 electrons. The first two electrons are located in the 1s orbital. In the second energy level, two electrons are .

An orbital is a wave function for an electron defined by the three quantum numbers, n, ℓ and m ℓ. Orbitals define regions in space where you are likely to find electrons. s orbitals (ℓ = 0) are spherical shaped. p orbitals (ℓ = 1) are dumb-bell shaped. The three possible p orbitals are always perpendicular to each other. According to Hund's rule, the sixth electron enters the second of those p orbitals and has the same spin as the fifth electron. 2.8: Electron Configurations is shared under a license and was authored, remixed, and/or curated by LibreTexts. There are a set of general rules that are used to figure out the electron configuration of an atomic .
NEW LEAKED. hungry4moore. meepmomentt
orbital notation for chlorine|chlorine electron configuration excited state